Target: HIF2α
Compound: NKT2152
Discovery
IND Enabling
Phase 1
Phase 2
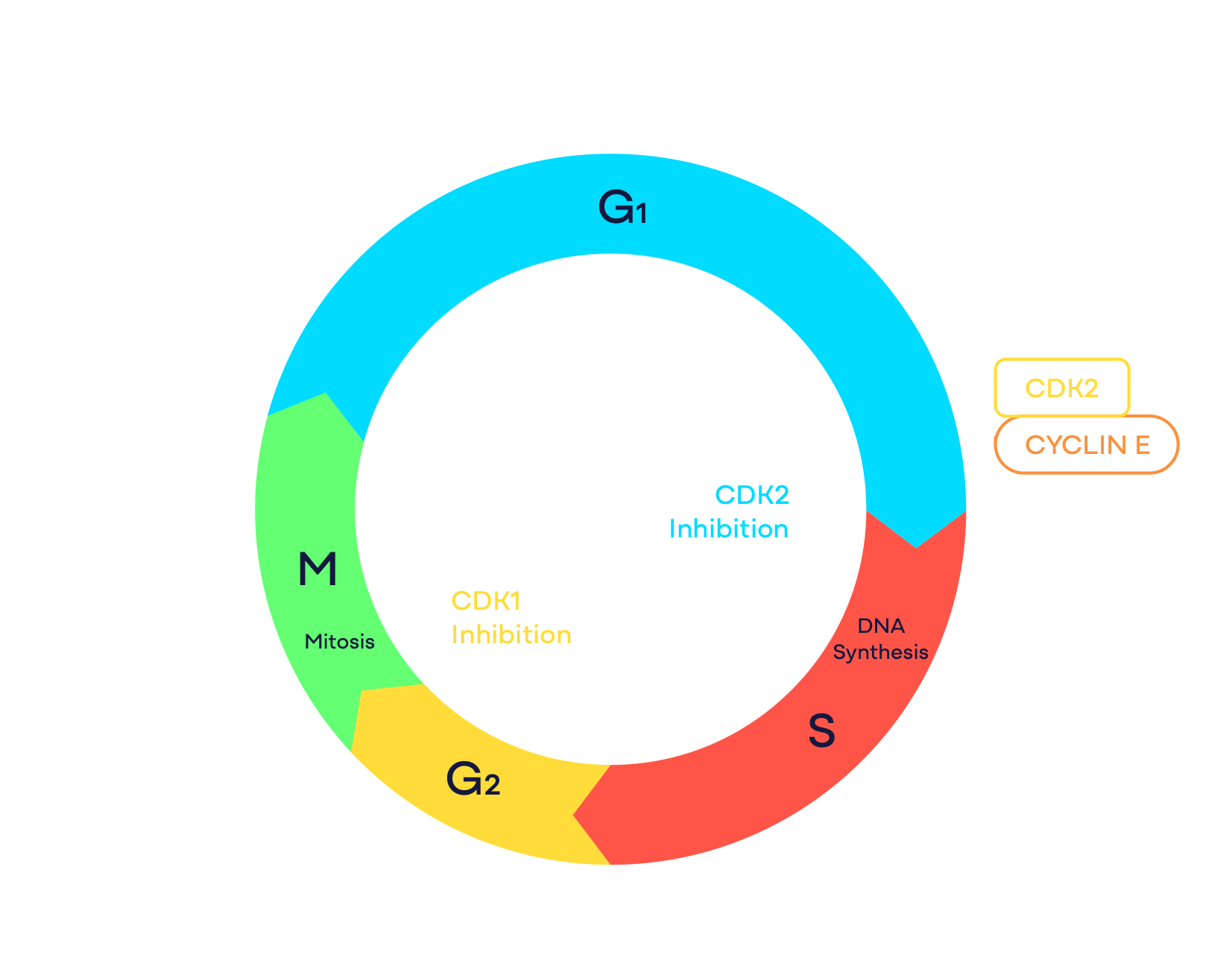
Target: CDK2
Compound: Small Molecule
Discovery
IND Enabling
Phase 1
Phase 2
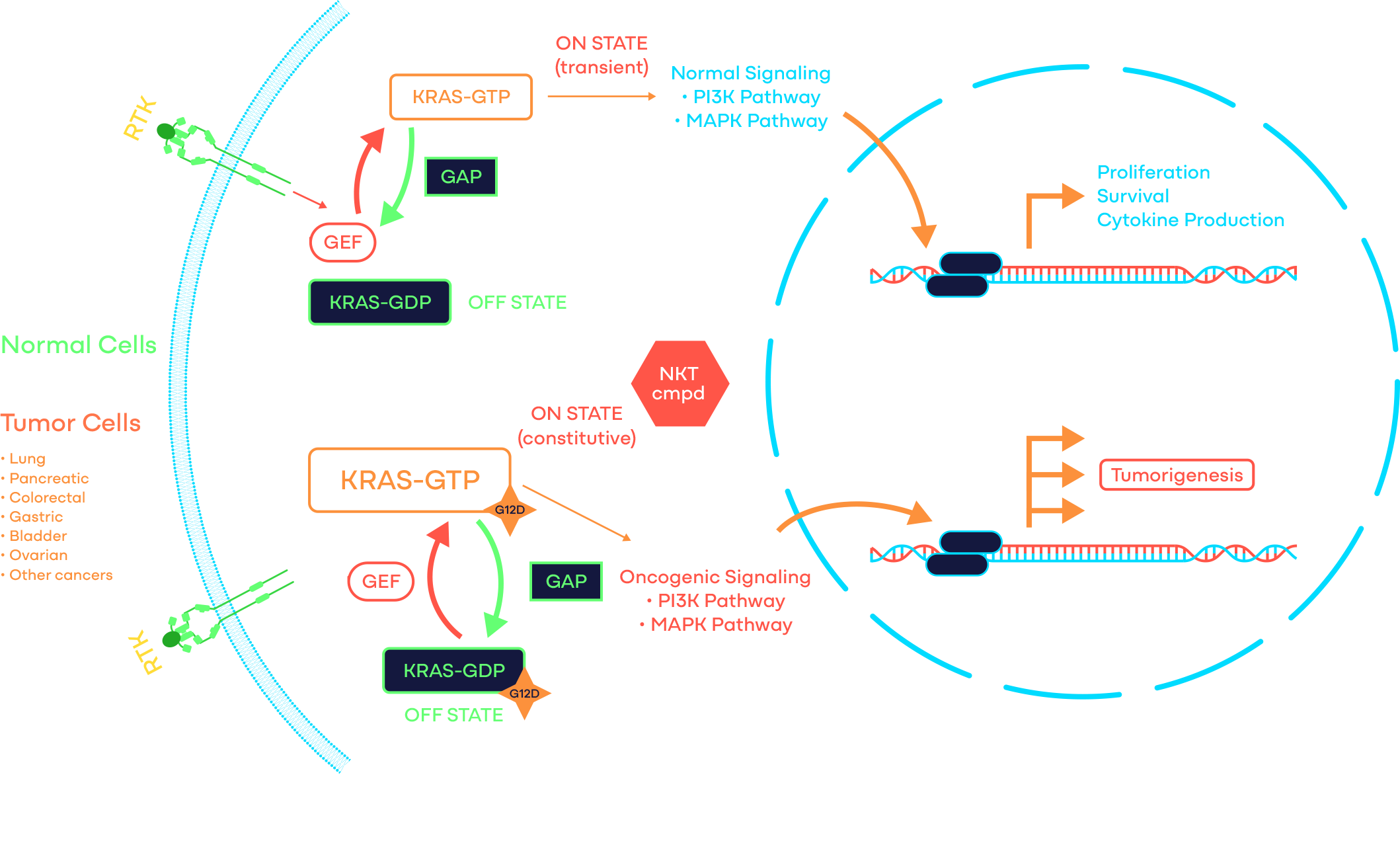
Target: KRAS G12D
Compound: Small Molecule
Discovery
IND Enabling
Phase 1
Phase 2
Target
Compound
Discovery
IND Enabling
Phase 1
Phase 2
HIF2α
NKT2152
CDK2
Small Molecule
KRAS G12D
Small Molecule
HIF2α Program
Hypoxia inducible factor 2α (HIF2α) is a transcription factor that regulates the expression of many genes, including those associated with angiogenesis, metabolism, cell proliferation, and migration etc. Under physiological oxygen condition, it is rapidly degraded through the von Hippel-Lindau (VHL, a tumor suppressor) E3 ligase complex. However, HIF2α is frequently stabilized and considered a validated target in VHL disease and clear cell renal cell carcinoma (ccRCC), which are characterized by germline and somatic VHL deficiency respectively. In addition, it is over-expressed in many solid tumors due to hypoxia, which invariably occurs when rapidly growing tumor outpaces the oxygen supply. Therefore, HIF2α antagonists may have a broad application beyond VHL disease and ccRCC. Furthermore, its restricted expression in normal tissue leads to a favorable toxicity profile for HIF2α inhibitors.
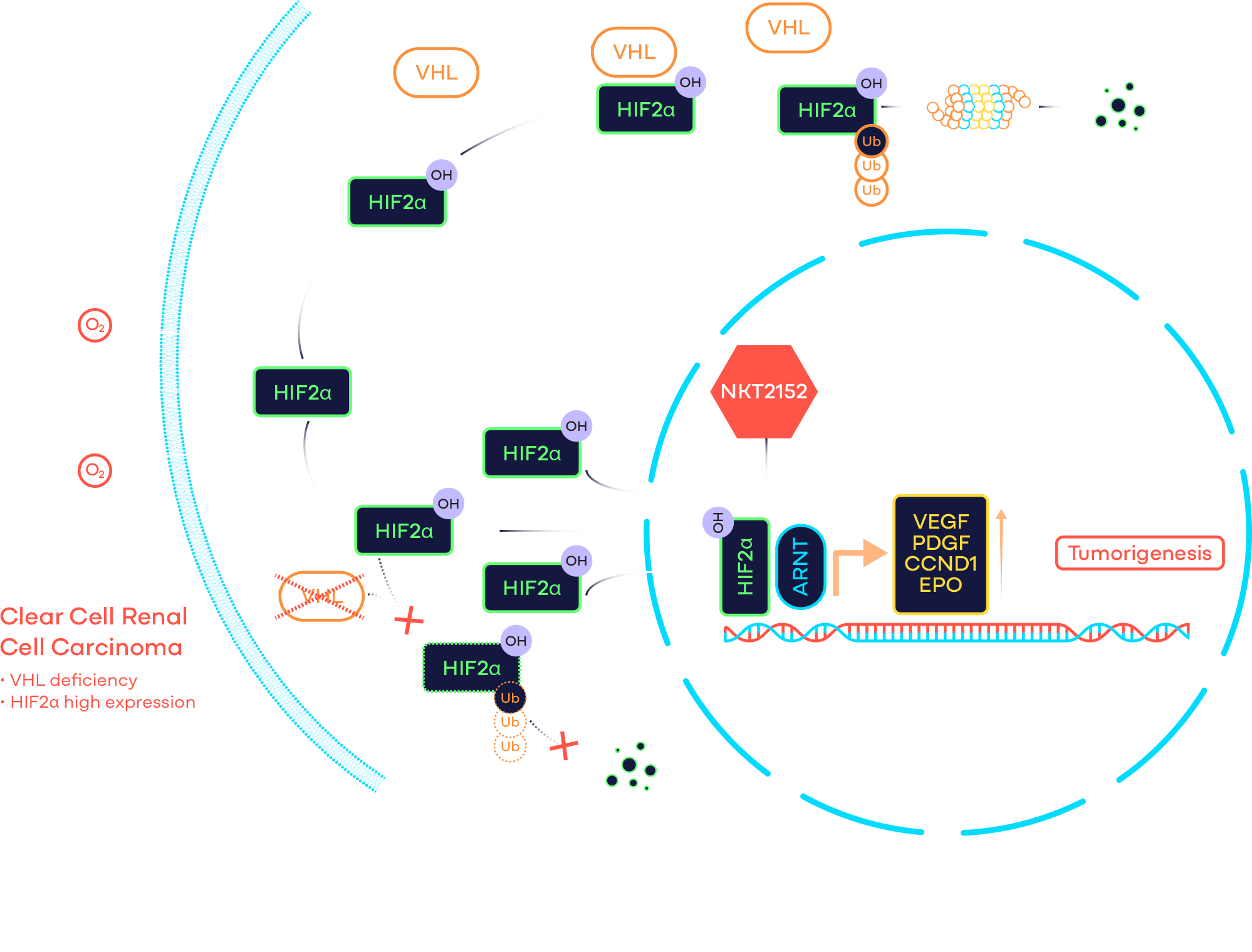
NKT2152 is a potent, selective, and orally available small molecule HIF2α inhibitor. Discovered by structure-based design approach, NKT2152 binds to HIF2α allosterically and disrupts HIF2α/HIF1β transcription factor complex. NKT2152 causes significant tumor growth inhibition or regression in multiple solid tumor xenograft models, including but not limited to ccRCC and hepatocellular carcinoma (HCC).
We are currently testing NKT2152 in a Phase 1/2 clinical study as a single agent (NCT05119335) and a Phase 2 ccRCC study in combination with palbociclib and sasanlimab (NCT05935748, Pfizer collaboration) for the treatment of ccRCC.
CDK2 Program
CDK2 is a cell cycle kinase that plays a critical role in controlling the G1 to S-phase cell cycle transition. Activation of CDK2 requires complex formation with cyclins including cyclin E or cyclin A. Abnormal activation of CDK2 through cyclin E1 amplification, loss of endogenous CDK/cyclin inhibitors p21/p27, or other mechanisms has been indicated as the primary oncogenic driver in advanced cancers including ovarian, endometrial, lung and gastric cancers. In addition, cyclin E1 overexpression has emerged as a key resistance mechanism to CDK4/6 inhibitors in hormone receptor-positive breast cancer patients. Based on mouse knockout studies, CDK2 does not play an essential role in embryo development and normal tissue homeostasis. Therefore, CDK2 represents a promising novel oncology target.
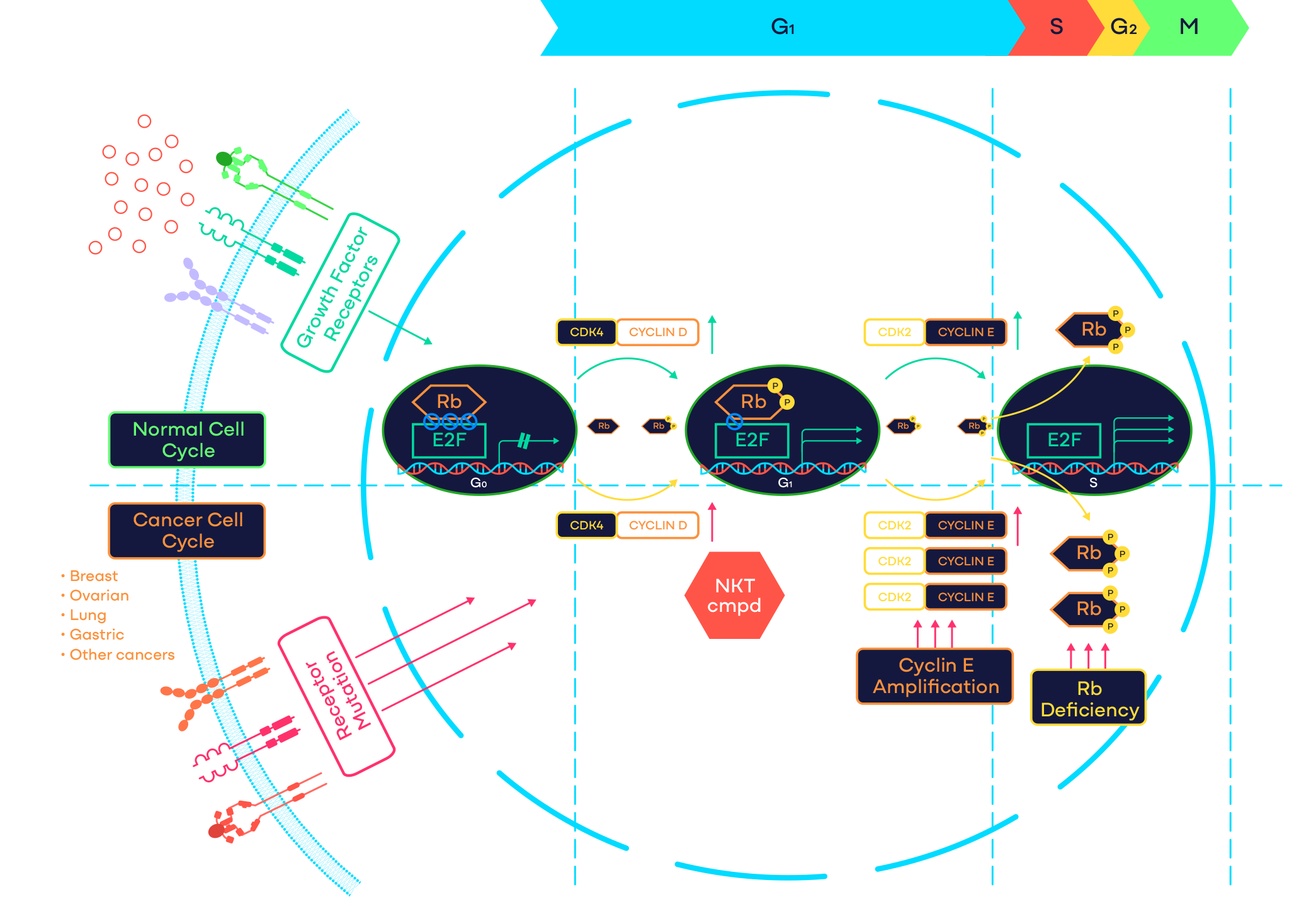
NKT3447 is an ATP competitive and reversible inhibitor of CDK2 with a prolonged target occupancy. Preclinical studies have shown that NKT3447 selectively suppresses the proliferation of cancer cells that depend on CDK2, while sparing normal cells. In animal models, NKT3447 has demonstrated sustained blockage of the CDK2 pathway, potent inhibition of tumor growth or regression.
NKT3447 is currently under evaluation in a Phase 1 clinical study in advanced or metastatic solid tumors as a single agent (NCT06264921).
NKT3964 is a first-in-class, highly potent and selective, orally bioavailable CDK2 PROTAC degrader, causing prolonged CDK2 pathway inhibition without cyclin E accumulation. It has the potential to maximally inhibit the CDK2 pathway, fully harnessing the therapeutic benefits of CDK2 inhibition.
NKT3964 is currently under evaluation in a Phase 1 clinical study in advanced or metastatic solid tumors as a single agent (NCT06586957).
KRAS G12D Program
KRAS is a signaling GTPase that cycles between a GDP-bound inactive and a GTP-bound active state. It is the most frequently mutated oncogene and G12D is the most common KRAS-activating mutation with > 68% of pancreatic cancers, > 40% of colorectal cancers, > 17% of lung adenocarcinomas, and many other cancers harboring KRAS G12D as one of the oncogenic drivers.
At NiKang, scientists are developing small molecule compounds to inhibit KRAS G12D to treat cancers driven by such mutations.